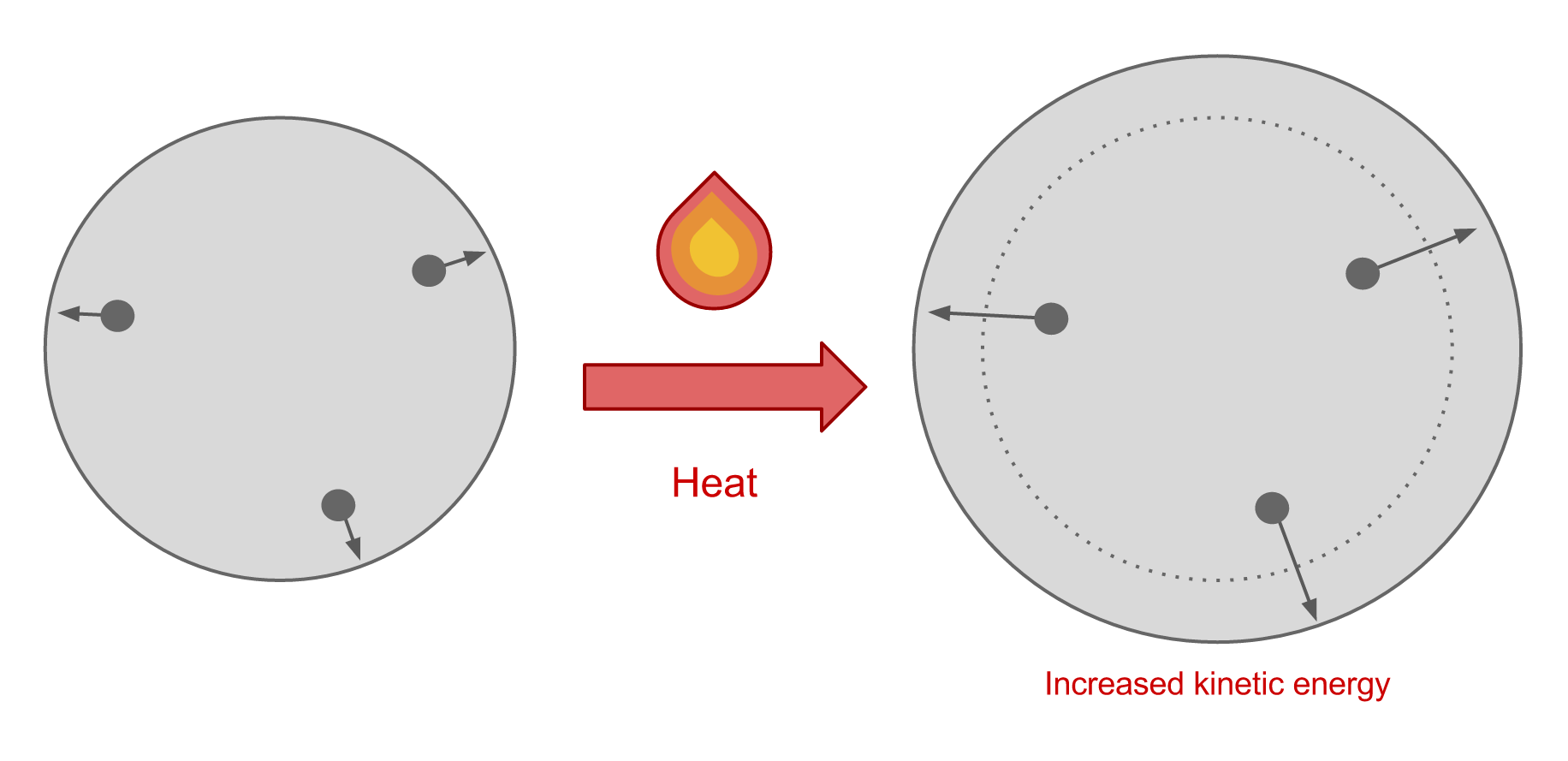
Why Does Gas Cool When It Expands . I simulate behavior of a gas in two chambers, one with higher. in this video i explain why expanding gas cools. the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. The work can be calculated in two ways because the. the internal energy of the system decreases as the gas expands.
from www.demos.smu.ca
expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. I simulate behavior of a gas in two chambers, one with higher. in this video i explain why expanding gas cools. The work can be calculated in two ways because the. the internal energy of the system decreases as the gas expands. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere.
Thermal Expansion/Contraction
Why Does Gas Cool When It Expands I simulate behavior of a gas in two chambers, one with higher. the internal energy of the system decreases as the gas expands. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. I simulate behavior of a gas in two chambers, one with higher. expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. The work can be calculated in two ways because the. in this video i explain why expanding gas cools. the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere.
From socratic.org
One "mol" of an ideal gas, initially at "300 K", is cooled at constant Why Does Gas Cool When It Expands in this video i explain why expanding gas cools. I simulate behavior of a gas in two chambers, one with higher. the internal energy of the system decreases as the gas expands. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). the reason gas. Why Does Gas Cool When It Expands.
From www.youtube.com
To show that solids, liquids, and gases expand on heating YouTube Why Does Gas Cool When It Expands The work can be calculated in two ways because the. the internal energy of the system decreases as the gas expands. the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. as a gas (like air) expands, the value of v increases and this has the effect of increasing. Why Does Gas Cool When It Expands.
From www.chegg.com
A Refrigerator Uses An Ideal Gas With Constant Vol... Why Does Gas Cool When It Expands when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. The work can be calculated in two ways because the. in this video i explain why expanding gas cools. the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. expansion. Why Does Gas Cool When It Expands.
From chem.libretexts.org
Chapter 11.1 Real Gases Chemistry LibreTexts Why Does Gas Cool When It Expands as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. I simulate behavior of a gas in two chambers, one with higher. the reason gas cools when it. Why Does Gas Cool When It Expands.
From www.aplustopper.com
Expansion and Contraction in Solids, Liquids and Gases A Plus Topper Why Does Gas Cool When It Expands expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. The work can be calculated in two ways because the. as a gas (like air) expands, the value of v increases and this has. Why Does Gas Cool When It Expands.
From socratic.org
How can heat cause motion in a liquid? + Example Why Does Gas Cool When It Expands I simulate behavior of a gas in two chambers, one with higher. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. The work can be calculated in two. Why Does Gas Cool When It Expands.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Why Does Gas Cool When It Expands as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). the internal energy of the system decreases as the gas expands. I simulate behavior of a gas in two chambers, one with higher. expansion of real gases lead to cooling as average translational kinetic energy per. Why Does Gas Cool When It Expands.
From www.vrogue.co
One Mole Of An Ideal Monoatomic Gas Expands Isotherma vrogue.co Why Does Gas Cool When It Expands expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. the internal energy of the system decreases as the gas expands. in this video i explain why expanding gas cools. as a. Why Does Gas Cool When It Expands.
From www.youtube.com
Heat is added to an ideal gas and the gas expands. In such a process Why Does Gas Cool When It Expands the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). the internal. Why Does Gas Cool When It Expands.
From www.youtube.com
Expansion of Air on Heating YouTube Why Does Gas Cool When It Expands the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. The work can be calculated in two ways because the. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). I simulate behavior of a gas in two chambers,. Why Does Gas Cool When It Expands.
From www.toppr.com
One mole of a van der Waals gas expands reversibly and isothermally at Why Does Gas Cool When It Expands when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). The work can be calculated in two ways because the. in this video i explain why expanding gas. Why Does Gas Cool When It Expands.
From saylordotorg.github.io
The Behavior of Real Gases Why Does Gas Cool When It Expands as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the temperature). The work can be calculated in two ways because the. in this video i explain why expanding gas cools. the internal energy of the system decreases as the gas expands. I simulate behavior of a gas. Why Does Gas Cool When It Expands.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Why Does Gas Cool When It Expands The work can be calculated in two ways because the. when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. in this video i explain why expanding gas cools. as a gas (like air) expands, the value of v increases and this has the effect of increasing t (the. Why Does Gas Cool When It Expands.
From www.numerade.com
SOLVED A volume of air (assumed to be an ideal gas) is first cooled Why Does Gas Cool When It Expands when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. I simulate behavior of a gas in two chambers, one with higher. expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. as a gas (like air) expands, the value of v increases and. Why Does Gas Cool When It Expands.
From www.numerade.com
SOLVED An ideal gas expands at a constant pressure of 6.00 105 Pa from Why Does Gas Cool When It Expands the reason gas cools when it expands is because it is expending energy by pushing against the atmosphere. I simulate behavior of a gas in two chambers, one with higher. in this video i explain why expanding gas cools. the internal energy of the system decreases as the gas expands. as a gas (like air) expands,. Why Does Gas Cool When It Expands.
From slideplayer.com
MATTER Solids, Liquids, & Gases I. States of Matter ppt download Why Does Gas Cool When It Expands the internal energy of the system decreases as the gas expands. The work can be calculated in two ways because the. I simulate behavior of a gas in two chambers, one with higher. when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. as a gas (like air) expands,. Why Does Gas Cool When It Expands.
From www.teachoo.com
Give reasons A gas exerts pressure on the walls of the container Why Does Gas Cool When It Expands expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. the internal energy of the system decreases as the gas expands. in this video i explain why expanding gas cools. I simulate behavior of a gas in two chambers, one with higher. the reason gas cools when it expands is because. Why Does Gas Cool When It Expands.
From www.demos.smu.ca
Thermal Expansion/Contraction Why Does Gas Cool When It Expands in this video i explain why expanding gas cools. expansion of real gases lead to cooling as average translational kinetic energy per molecule decreases. when cooled, the kinetic energy of the gas molecules decreases, meaning they move more slowly and have less. the reason gas cools when it expands is because it is expending energy by. Why Does Gas Cool When It Expands.